Chemistry is the scientific research of matter, together with its construction, properties, and the modifications it undergoes. On the basis of chemistry lies the idea of atoms and parts. These basic items play an important position within the chemistry of life, notably in understanding the composition and performance of the human physique. This be aware explores the essential construction of atoms, the excellence between atoms and parts, the most important parts within the human physique, and the position of isotopes and radioisotopes in chemistry.
Atoms and Atomic Construction
Atoms are the smallest items of matter that retain the chemical properties of a component. They include three major subatomic particles: protons, neutrons, and electrons.
- Protons are positively charged particles situated within the atomic nucleus. They’ve a mass of roughly 1 atomic mass unit (amu). The variety of protons in an atom defines the ingredient and is called the atomic quantity. For instance, all carbon atoms have 6 protons.
- Neutrons are impartial particles (i.e., they don’t have any cost) that additionally reside within the nucleus. They’re barely bigger than protons and have a mass of about 1 amu. Neutrons contribute to the atomic mass however don’t have an effect on the atom’s cost.
- Electrons are negatively charged particles with negligible mass in comparison with protons and neutrons. They orbit the nucleus in areas referred to as electron shells. Regardless of their minimal mass, electrons are essential in chemical bonding and reactions.
The nucleus, which comprises protons and neutrons, is extremely dense in comparison with the electron shells, that are comparatively huge. Though electrons are unfold over a big quantity, most of an atom’s mass is concentrated within the nucleus.
Electron Shells
The classical mannequin of the atom depicts electrons orbiting the nucleus in a way much like planets across the solar. Nevertheless, this can be a simplification. Electrons exist in areas of chance referred to as electron shells. The primary shell can maintain as much as 2 electrons, the second shell as much as 8, and the third shell as much as 18. Electrons fill the bottom power ranges (or shells) first earlier than occupying greater ones. This association influences how atoms work together chemically.
Atoms vs. Components
The phrases “atom” and “element” are sometimes used interchangeably however have distinct meanings:
- Atom: An atom is the smallest unit of a component that retains its chemical properties. It consists of protons, neutrons, and electrons. Atoms can mix to kind molecules, that are the constructing blocks of matter.
- Ingredient: A component is a pure substance consisting of just one sort of atom. Components are outlined by their atomic quantity, which is the variety of protons of their atoms. For example, hydrogen (H) is a component with atoms which have one proton. There are presently 118 recognized parts, every with distinctive properties.
Main Components within the Human Physique
The human physique is primarily composed of some key parts, that are important for varied organic capabilities:
- Oxygen (O): Making up about 65% of the human physique by mass, oxygen is essential for mobile respiration, the method by which cells generate power.
- Carbon (C): Comprising roughly 18% of the physique, carbon is the spine of natural molecules, together with carbohydrates, proteins, lipids, and nucleic acids.
- Hydrogen (H): Accounting for about 10% of the physique, hydrogen is a part of water and natural molecules, enjoying an important position in sustaining pH stability and power manufacturing.
- Nitrogen (N): Making up about 3% of the physique, nitrogen is a key ingredient in amino acids, that are the constructing blocks of proteins, and in nucleic acids, which make up DNA and RNA.
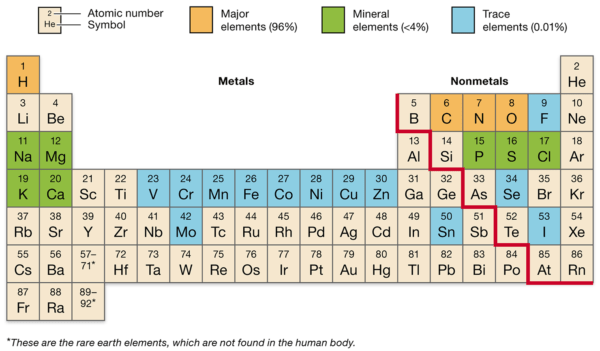
Different parts, resembling calcium, phosphorus, potassium, sulfur, sodium, and magnesium, are current in smaller portions however are nonetheless important for sustaining well being and supporting varied physiological capabilities.
Atomic Quantity, Mass Quantity, Isotopes, and Radioisotopes
To know the variation amongst atoms, we have to distinguish between atomic quantity, mass quantity, isotopes, and radioisotopes:
- Atomic Quantity: The atomic quantity is the variety of protons within the nucleus of an atom. It defines the ingredient and its place on the periodic desk. For example, the atomic variety of carbon is 6, which means all carbon atoms have six protons.
- Mass Quantity: The mass quantity is the sum of protons and neutrons in an atom’s nucleus. It represents the atom’s complete nuclear mass. For instance, carbon-12 has a mass variety of 12, indicating a complete of 6 protons and 6 neutrons.
- Isotopes: Isotopes are variants of the identical ingredient which have the identical variety of protons however completely different numbers of neutrons. This leads to completely different mass numbers. For instance, carbon has three isotopes: carbon-12 (6 protons and 6 neutrons), carbon-13 (6 protons and seven neutrons), and carbon-14 (6 protons and eight neutrons).
- Radioisotopes: Radioisotopes, or radioactive isotopes, are unstable isotopes that decay over time, emitting radiation within the course of. This radioactive decay modifications the isotope right into a extra secure kind. Radioisotopes are utilized in varied functions, together with medical diagnostics and therapy. For instance, iodine-131 is utilized in treating thyroid problems.
Manufacturing of Isotopes
Isotopes are produced by varied nuclear processes:
- Pure Processes: Some isotopes happen naturally. For example, carbon-14 is constantly shaped within the ambiance by the interplay of cosmic rays with nitrogen-14.
- Synthetic Processes: Isotopes can be artificially created in laboratories or nuclear reactors. By bombarding secure atoms with neutrons or different particles, scientists can produce isotopes with completely different mass numbers. For example, technetium-99m, utilized in medical imaging, is produced in nuclear reactors.
Conclusion
Understanding the essential ideas of atomic construction, the excellence between atoms and parts, and the roles of isotopes and radioisotopes is prime in chemistry and important for exploring the chemistry of life. These ideas assist elucidate the composition and performance of organic molecules and using radioactive supplies in medication. By greedy these rules, we achieve insights into how the smallest items of matter contribute to the advanced processes that maintain life.

