Researchers have found how a cell floor protein known as Aplp1 can play a job in spreading materials chargeable for Parkinson’s illness from cell-to-cell within the mind.
Promisingly, an FDA-approved most cancers drug that targets one other protein known as Lag3 – which interacts with Aplp1 – blocks the unfold in mice, suggesting a possible remedy might exist already.
In a paper revealed final yr, a world workforce of scientists describes how the 2 proteins work collectively to assist dangerous alpha-synuclein protein clumps get into mind cells.
“Now that we know how Aplp1 and Lag3 interact, we have a new way of understanding how alpha-synuclein contributes to the disease progression of Parkinson’s disease,” neuroscientist Xiaobo Mao from Johns Hopkins College stated in June.
“Our findings also suggest that targeting this interaction with drugs could significantly slow the progression of Parkinson’s disease and other neurodegenerative diseases.”
Greater than 8.5 million individuals globally have Parkinson’s, the second commonest neurodegenerative illness after Alzheimer’s.
As a progressive motion dysfunction, it is often solely recognized when signs present, which embody tremors, stiffness, stability issues, speech difficulties, disturbed sleep patterns, and psychological well being points. Presently incurable, the illness means sufferers might finally battle to stroll or converse.
Parkinson’s signs primarily end result from the loss of life or impairment of dopamine-producing neurons within the mind’s substantia nigra, a area concerned in fantastic motor management. That is considered brought on by Lewy our bodies, that are irregular clumps of protein largely consisting of misfolded alpha-synuclein that journey between neurons.
Alpha-synuclein sometimes maintains practical communication between neurons, however issues come up when it turns into misfolded and insoluble. That stated, figuring out whether or not that is a reason for Parkinson’s or a symptom is troublesome.
Previous research on mice discovered Lag3 binds to alpha-synuclein proteins and spreads Parkinson’s illness pathology in neurons. Whereas deleting Lag3 considerably impedes this course of, it doesn’t fully stop it, indicating one other protein was additionally implicated in neurons taking in misfolded alpha-synuclein.
“Our work previously demonstrated that Lag3 wasn’t the only cell surface protein that helped neurons absorb alpha-synuclein, so we turned to Aplp1 in our most recent experiments,” stated Johns Hopkins neuroscientist Valina Dawson.
The scientists carried out exams with genetically modified mice that had been lacking both Aplp1 or Lag3, or each. They discovered Aplp1 and Lag3 can every independently assist mind cells soak up dangerous alpha-synuclein, however collectively they considerably enhance the uptake.
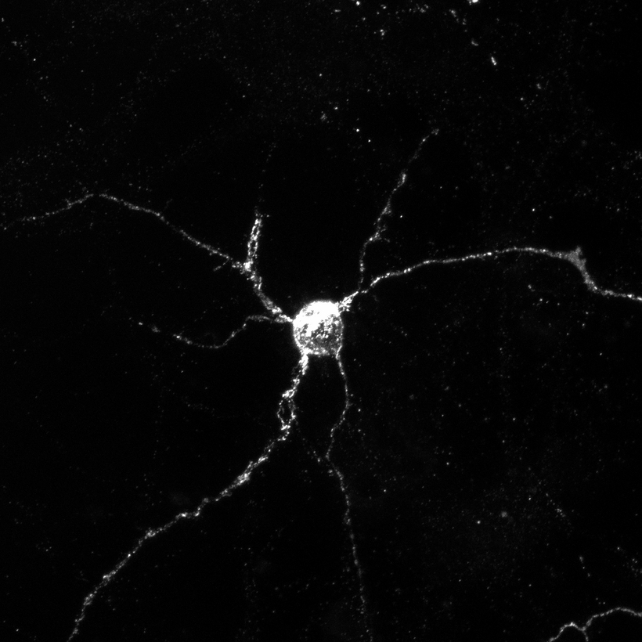
When mice had been lacking each Aplp1 and Lag3, 90 p.c much less of the dangerous alpha-synuclein entered wholesome mind cells, that means a higher quantity of the dangerous protein clumps was blocked with each proteins lacking in contrast with a deletion of only one.
The researchers gave regular mice the drug nivolumab/relatlimab, a melanoma remedy that comprises a Lag3 antibody, and located that it additionally stopped Aplp1 and Lag3 from interacting, once more virtually fully blocking the formation of disease-causing alpha-synuclein clumps in neurons.
“The anti-Lag3 antibody was successful in preventing further spread of alpha-synuclein seeds in the mouse models and exhibited better efficacy than Lag3-depletion because of Aplp1’s close association with Lag3,” stated Ted Dawson, a neuroscientist at Johns Hopkins College.
The subsequent step will likely be to check the Lag3 antibody on mouse fashions of Parkinson’s illness and Alzheimer’s – the place analysis has pointed to Lag3 as a goal too.
The analysis has been revealed in Nature Communications.
An earlier model of this text was revealed in June 2024.

